In Las Vegas, Lazaro Hernandez, a high-profile poker player known for his extravagant lifestyle and televised tournament appearances with colorful stacks of chips, was concealing a dangerous secret.
Federal investigators allege that Hernandez was involved in a massive $230 million scheme to counterfeit prescription medications, especially crucial HIV drugs.
His operation involved altering pill bottles and selling these manipulated drugs back to pharmacies at steep discounts.
Court records indicate that Hernandez’s counterfeit operation included altering bottles for Biktarvy, the leading prescribed HIV medication, as well as Descovy, another HIV drug, among other pharmaceuticals.
In some instances, the pills inside the bottles were replaced with Seroquel, an antipsychotic medication.
Based in South Florida, Hernandez used the proceeds from his counterfeiting scheme to fund his gambling, frequently taking private jets to Las Vegas and participating in numerous poker tournaments, according to authorities.
This drug counterfeiting operation contributes to what the World Health Organization estimates as up to $431 billion worth of counterfeit drugs globally each year.
In the U.S., there were 2,121 incidents of drug counterfeiting in 2022, marking a 17% increase from the previous year, according to the Pharmaceutical Security Institute, which monitors industry trends.
This issue is a major concern for Gilead Sciences, which has prioritized combating prescription drug diversion and counterfeiting.
In July 2021, Gilead filed a lawsuit against 161 defendants, including pharmacies and pharmaceutical distributors, accusing them of participating in a scheme to alter and resell its medications, Biktarvy and Descovy. S
imilarly, Johnson & Johnson filed a lawsuit in April 2022 against 27 defendants over its HIV medication Symtuza. The suits are ongoing.
“These criminals are exploiting the most vulnerable,” said Lori Mayall, Gilead’s head of anti-counterfeiting and product security.
Mayall elaborated on what constitutes counterfeit medicine during an interview at Gilead’s headquarters in Foster City, California.
Counterfeit drugs may involve altered packaging, incorrect tablets in bottles, incorrect caps or labels, and even altered leaflets containing essential medication information.
Drug diversion typically involves a patient who fills a prescription for an expensive medication covered by Medicare, Medicaid, or insurance. The patient then sells the medication for a fraction of its list price.
An aggregator buys the medication, removes patient information, alters the bottle, and sells it to a wholesale distributor, who then resells it to pharmacies.
Biktarvy has a list price of $3,795, though patient copays are often much lower, and discounts are available through Gilead’s patient assistance programs.
Gilead discovered its counterfeit issue in August 2020, when an independent pharmacy reported receiving a sealed bottle of Biktarvy containing Excedrin pills instead.
Initially, the bottle and label appeared genuine, but over the following months, more complaints emerged about sealed bottles of Biktarvy containing Seroquel.
Counterfeiters had used authentic empty bottles, filled them with incorrect pills, and repackaged them with counterfeit seals. One patient reportedly suffered temporary paralysis and speech loss after taking Seroquel but recovered soon after.
Mayall noted that counterfeiters reused bottles, cleaned and repackaged them to mimic genuine Gilead products.
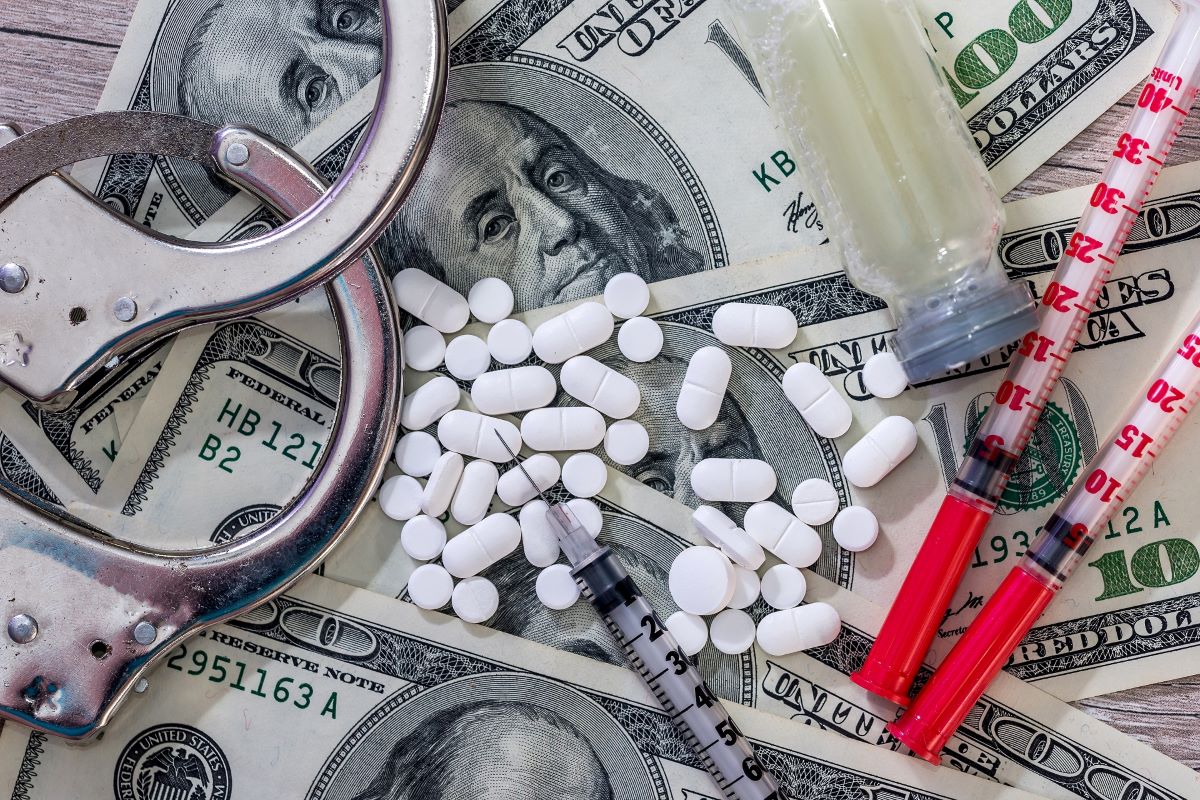
Under the federal Drug Supply Chain Security Act, every prescription medication sale is supposed to be tracked to maintain a chain of custody back to the manufacturer.
However, criminals like Hernandez have managed to bypass these safeguards by altering labels and prescription paperwork and falsifying supply chain documentation, according to law enforcement officials.
Drug diversion often begins at the street level, where patients are approached outside shelters or clinics and paid to sell their medications, which are then cleaned, repackaged, and resold.
At Gilead’s headquarters, a “war room” contains thousands of confiscated counterfeit pills and bottles. Some fakes are evident due to misspellings on the paperwork.
Legitimate Gilead drugs are sold to licensed distributors, who then supply pharmacies. Counterfeit medications related to the Hernandez case were repackaged to look like genuine Gilead products.
The Gilead lawsuit includes four licensed distributors: Safe Chain Solutions, Scripts Wholesale, ProPharma Distribution, and ProVen.
Safe Chain Solutions responded denying any involvement in selling counterfeit drugs and claiming it was a victim of the conspiracy. ProPharma Distribution settled the lawsuit for $3.3 million and agreed to stop selling Gilead medications. Details of the settlement remain confidential.
Johnson & Johnson learned in November 2020 that counterfeit versions of its HIV medication Symtuza were being distributed to U.S. pharmacies and reported it to the FDA.
“Counterfeiting life-saving medications is a criminal act that endangers patient lives,” said Dr. Dave Anderson, a company vice president.
Geoffrey Potter, a partner at Patterson Belknap Webb & Tyler, who represents both Gilead and Johnson & Johnson, noted that the extent of counterfeit medications in the system is difficult to measure, but identified schemes are often large.
He explained that distinguishing real pills from counterfeits can be challenging because most people do not inspect their medications closely.
Stephen Mahmood, assistant special agent in charge at the U.S. Department of Health and Human Services’ Office of Inspector General, expressed concern about the widespread nature of drug diversion fraud. Some pharmacies might unknowingly receive counterfeit drugs, while others might be complicit.
In a 2014 Miami case, HHS-OIG agents filmed individuals altering medication bottles using lighter fluid to remove patient information. Those individuals were convicted in 2015 and served prison time.
A convicted felon known as “Julio” recounted his decade-long involvement in the counterfeit drug business, describing how he paid patients for their medications, cleaned and repackaged the bottles, and sold them to wholesalers who then resold them to pharmacies.
The FDA urges the public to obtain prescription drugs only from state-licensed U.S. pharmacies or physicians to ensure drug quality and safety.
Hernandez’s high-flying lifestyle ended abruptly when he pleaded guilty in April to conspiracy charges related to distributing adulterated and misbranded drugs and money laundering in connection with the $230 million fraud ring.
He was sentenced to 15 years in prison in June. His attorneys noted his gambling addiction as a significant factor in his criminal activities.
None of the distributors in the Gilead case have faced criminal charges, although Steven Diamanstein of Scripts Wholesale was indicted for buying and reselling illegally diverted prescription HIV medication. He has pleaded not guilty.
Further investigations into counterfeit schemes are ongoing, with authorities emphasizing the need to prevent these crimes and secure the drug supply chain.






Leave a Reply