On Wednesday, Pfizer reported strong results from a mid-stage clinical trial of its experimental vaccine targeting Group B Streptococcus (GBS), a potentially deadly bacterial disease.
This development marks a significant step forward as the vaccine progresses toward potential approval.
Pfizer is one of several pharmaceutical companies striving to create the world’s first vaccine against Group B strep, a disease responsible for nearly 150,000 infant deaths globally each year, particularly in lower-income countries.
In September, the Food and Drug Administration (FDA) granted breakthrough therapy designation to Pfizer’s vaccine, a status aimed at speeding up the development and review process for promising drugs.
The single-dose vaccine produced antibodies that may offer infants significant protection against GBS, according to data from a phase two clinical trial released on Wednesday.
The vaccine is administered to expectant mothers, who then pass the vaccine-induced antibodies to their fetuses. This maternal vaccination approach is similar to the one Pfizer uses for its respiratory syncytial virus (RSV) vaccine.
Pfizer’s positive phase two results raise hopes that maternal vaccination against GBS could prevent thousands of infant cases. These findings will also inform the planning of phase three clinical trials, which are typically necessary for FDA approval.
The Bill & Melinda Gates Foundation, which supported the phase two trial, awarded Pfizer an additional $100 million grant last year. This funding will support late-stage trials and help ensure that the vaccine reaches lower-income countries if it gains approval.
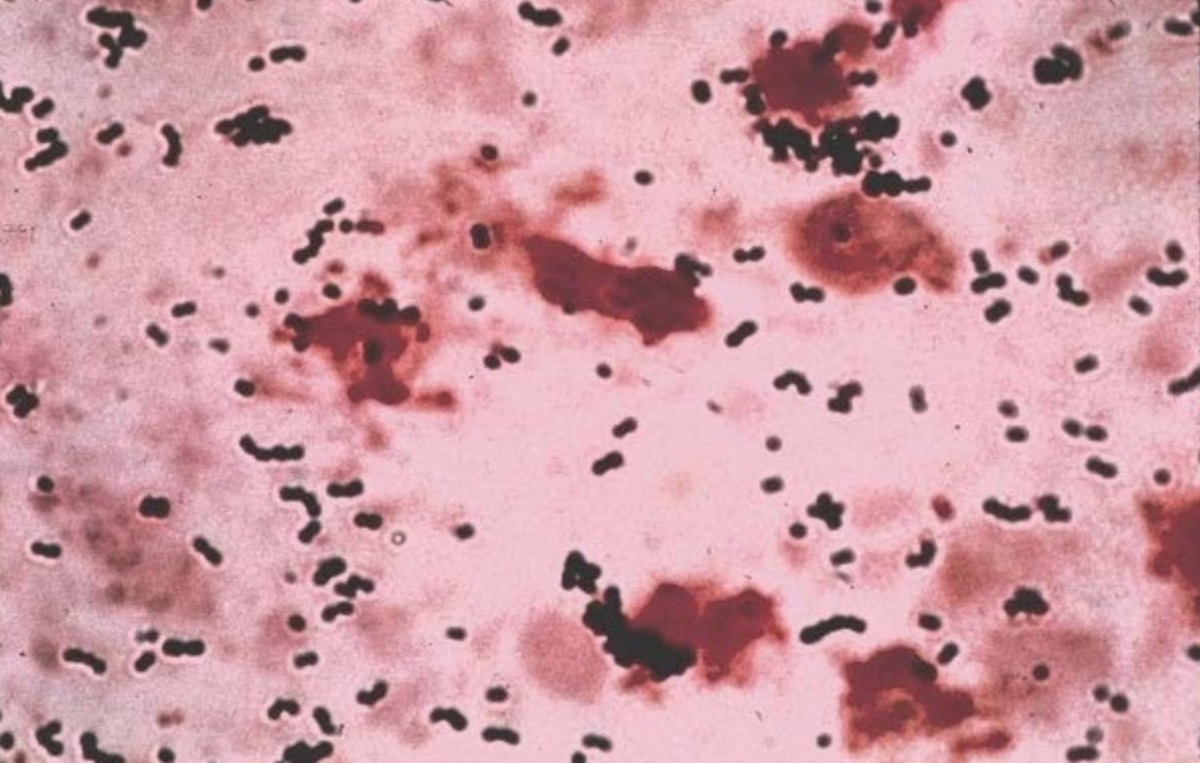
GBS disease, caused by a common bacterium that many adults carry without harm, can be passed from mother to newborn during labor and delivery.
This can lead to severe infections in the baby during the first few weeks or months of life. According to the Centers for Disease Control and Prevention (CDC), about one in four women carry GBS bacteria.
Infants with GBS infections can exhibit symptoms such as fever and difficulty breathing. Invasive GBS infections can cause serious complications like pneumonia, bloodstream infections, and meningitis.
There are 10 different GBS serotypes, or variations of the bacteria. Pfizer’s vaccine targets six of the most prevalent serotypes, which together account for 98% of GBS cases worldwide.
In the trial, 360 healthy pregnant individuals in South Africa were randomly assigned to receive a single shot at three different dosage levels, either with or without a specific adjuvant, or a placebo.
The trial found that Pfizer’s vaccine generated strong antibodies against the six GBS serotypes in the mothers. These antibodies were “efficiently transferred” to infants, with transfer ratios ranging from 0.4 to 1.3, depending on the dose.
This means that some infants received only a fraction of the antibodies their mothers had, while others received higher antibody levels than their mothers.
Pfizer indicated that these antibody transfer levels are associated with a reduced risk of GBS, a conclusion supported by a parallel natural history study conducted in South Africa.
The trial results suggested that the safety profile for both mothers and infants was comparable between the vaccine and placebo groups, indicating that the shot was generally well tolerated.
Post-vaccination reactions in mothers were typically mild or moderate and short-lived. About 2% to 8% of participants who received the vaccine reported fever, compared with 5% in the placebo group.
Between 45% and 70% of vaccinated pregnant individuals experienced adverse reactions such as headache and vomiting, but the placebo group showed similar rates, with more than 60% reporting these events.
Among infants, 62% to 75% in the vaccine group and 74% in the placebo group experienced adverse events, including upper respiratory tract infections.
There were three infant deaths in the vaccine group and two in the placebo group, but the study authors concluded that none of these events were related to the vaccine.
These results emerge as Pfizer anticipates a continued decline in Covid-related sales this year.
The company also faces a patent cliff, with the loss of market exclusivity for several blockbuster drugs like the cancer treatments Xtandi and Ibrance expected to impact its annual revenues by 2030.
To mitigate a sharp drop in sales, Pfizer is shifting its focus toward developing a new drug pipeline and pursuing mergers and acquisitions.





Leave a Reply