The Food and Drug Administration (FDA) has recalled over half a million Covid tests due to concerns about bacterial contamination.
The FDA announced that it “has significant concerns of bacterial contamination” in the test kit solution of certain Pilot COVID-19 At-Home Tests. The use of this contaminated solution “may pose safety concerns” and could lead to inaccurate test results.
More than 500,000 tests were distributed to CVS drugstores, while approximately 16,000 were sent to Amazon. None of these tests were part of any federal government testing programs.
To identify contaminated tests, consumers can check the lot numbers listed on the FDA’s website.
The contamination was found in the liquid of the test kits, which was contaminated with bacteria such as Enterococcus, Enterobacter, Klebsiella, and Serratia species.
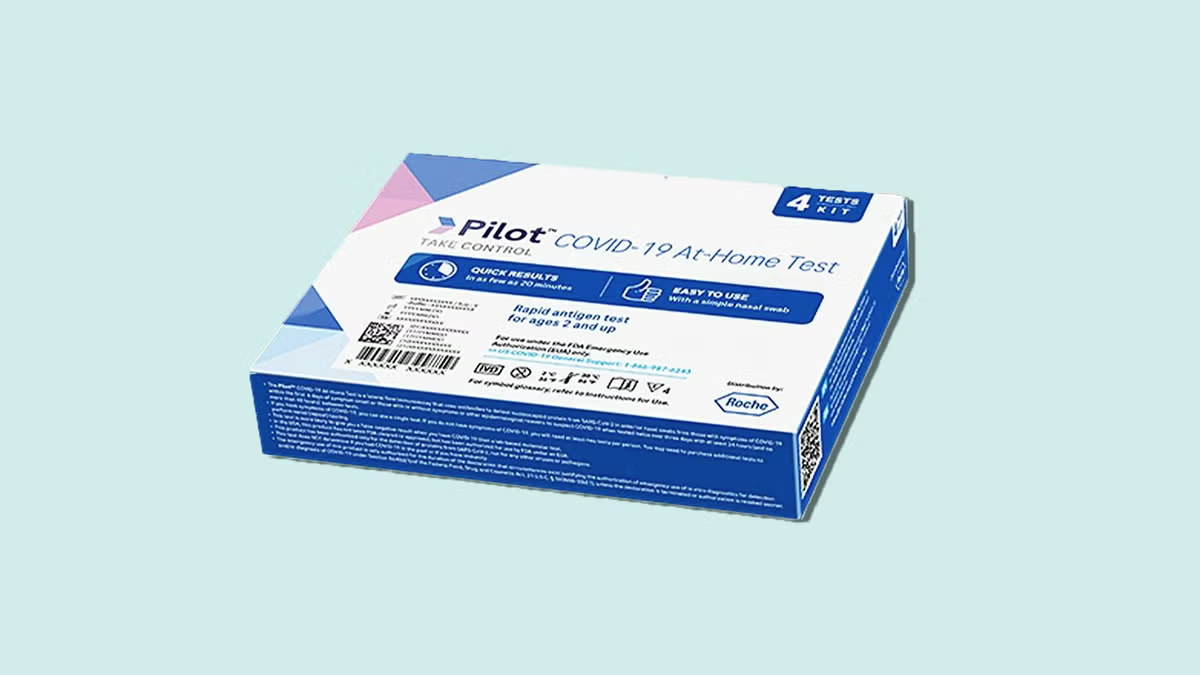
These bacteria could potentially cause illness, particularly in individuals with weakened immune systems, the FDA stated.
The FDA recommended that individuals watch for “signs of bacterial infection,” which might include “fever, discharge, red eyes, or any other concerning symptoms.”
Evie Baik, a spokesperson for SD Biosensor said that the bacteria likely originated from raw materials supplied by one of their suppliers. SD Biosensor has since severed ties with this supplier and is enhancing its quality control measures.
Roche, the distributor for SD Biosensor, mentioned that the issue “was identified during routine quality assurance testing,” as reported by CBS News.
Both SD Biosensor and Roche are cooperating with the FDA and are working with distributors and retailers to hold back the affected tests while the investigation continues.





Leave a Reply