Now that we have become accustomed to doing nose swabs for COVID-19 tests, we will soon have the option to test for the flu simultaneously.
The Food and Drug Administration has authorized the first over-the-counter at-home test that can detect and differentiate between flu and COVID-19.
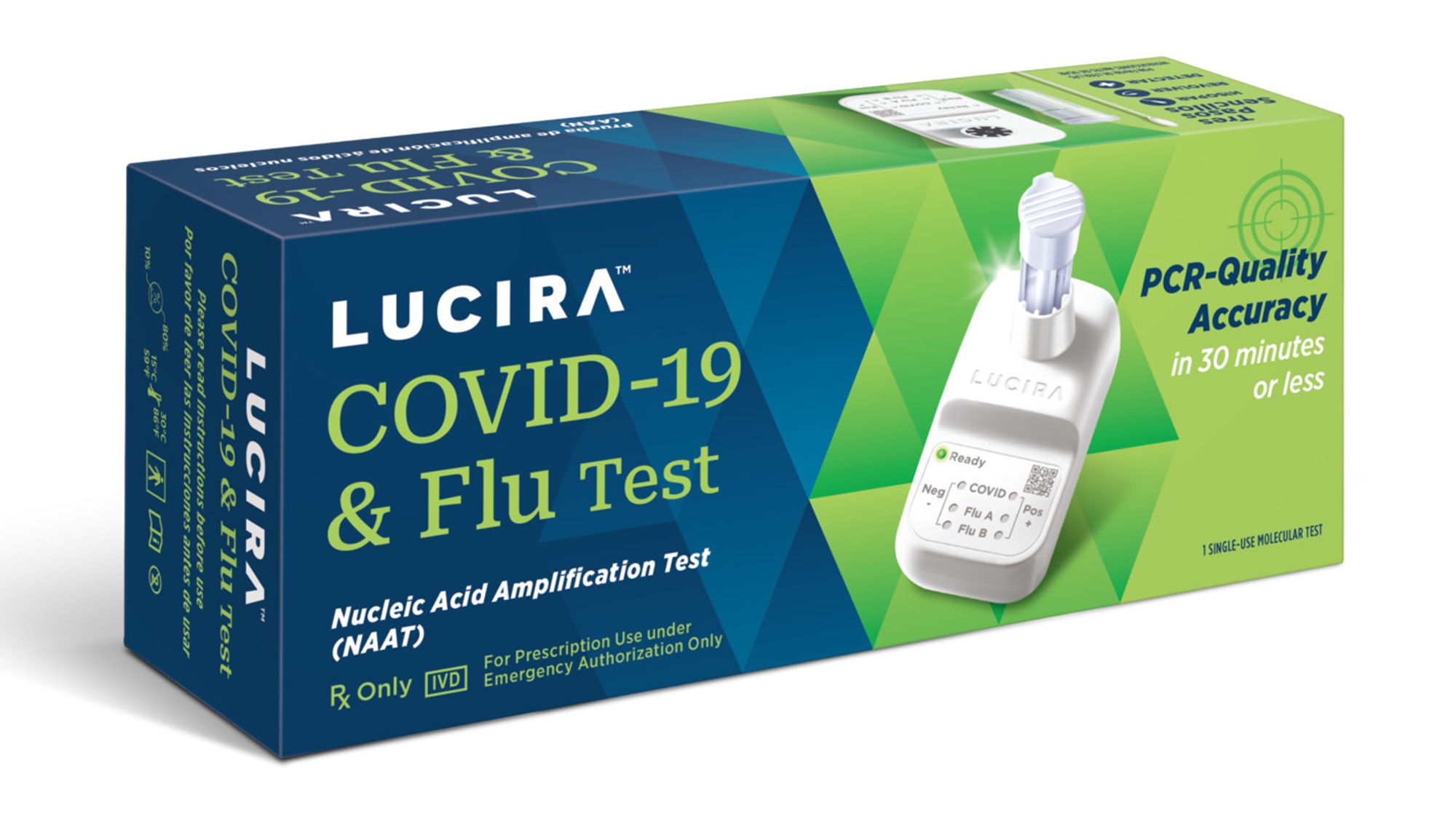
The Lucira COVID-19 and Flu Home Test is a single-use test that can be bought without a prescription. It uses a nasal swab, similar to at-home COVID tests, and displays results in 30 minutes or less, indicating positive or negative for influenza A, influenza B, and COVID-19.
“Today’s authorization of the first OTC test that can detect influenza A and B, along with SARS-CoV-2, is a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home,” said Dr. Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, in the announcement Friday.
Lucira Health started offering its COVID-19 and flu test in Canada in August 2022, priced at about $70. Insurance coverage may also impact how much consumers pay.
Lucira Health will announce the U.S. price and release date later, according to company president and CEO Erik Engelson.
“This is a major milestone for Lucira Health and at-home diagnostics, and I can’t thank our employees and partners enough for seeing this through, and of course, for the FDA’s recognition,” he said.
The company also sells the Lucira Check It COVID-19 Test for $34.99, a molecular test similar to polymerase chain reaction (PCR) lab tests and considered more accurate than other at-home rapid antigen tests.
In addition to Canada, at-home tests for COVID and flu are available in Australia and Europe, where they also test for RSV.
The Lucira COVID-19 and Flu Home Test is a single-use test that can be purchased without a prescription.
Using a nasal swab, the test displays results in 30 minutes or less, indicating positive or negative for influenza A, influenza B, and COVID-19.
How accurate is the at-home COVID and flu test?
The FDA said the test correctly identified:
– 100% of negative and 88.3% of positive COVID-19 samples
– 99.3% of negative and 90.1% of positive Influenza A samples
– 99.9% of negative Influenza B samples.
Lucira Health, a medical technology company based in Emeryville, California, had hoped to receive an emergency use authorization from the FDA around the same time it got approval from Canada.
It had already begun manufacturing tests to meet anticipated demand as demand for its COVID-19 test declined. Without the expected revenue from the combo test, the company filed for Chapter 11 bankruptcy protection and plans to pursue a sale of the business, it announced on February 22.







Leave a Reply